Abstract
Introduction
Post-transplant lymphoproliferative disease (PTLD) is a rare and life-threatening complication of solid organ transplantation (SOT). Most are of B-cell origin and treated with rituximab with or without chemotherapy. Patients achieving a complete response (CR) to rituximab have good outcomes and can avoid the excess toxicity of chemotherapy. The prospective PTLD-1 trial reported a CR rate of 25% with rituximab. We postulated adding ibrutinib to rituximab may increase CR rates, avoid chemotherapy for more patients and improve outcomes overall.
Methods
TIDaL is a prospective, single-arm, phase II trial evaluating the activity of ibrutinib in combination with rituximab (IR), with chemotherapy added using a risk-stratified sequential treatment strategy. Eligible patients had treatment naïve, CD20-positive PTLD of any histologic subtype arising after SOT. Patients required measurable disease and adequate organ function.
All patients received ibrutinib 560 mg daily with 4 doses of rituximab 375 mg/m 2 on days 1, 8, 15 and 22. Interim response to IR was assessed by CT scan between days 42-49. Risk stratification for subsequent therapy was based on baseline and interim CT response. Patients were assigned to the low risk arm if they achieved a CR on interim CT (irrespective of IPI) or a partial response (PR) with an IPI of 0-1. They continued with IR receiving 4 further 3-weekly doses of rituximab, ibrutinib continued until 3 weeks after the last dose of rituximab. All other patients were assigned to the high risk arm and received 4 cycles of R-CHOP-21 (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone in 21-day cycles). Ibrutinib continued until 3 weeks after the last cycle of R-CHOP for all , . Prophylaxis against Pneumocystis pneumonia was mandated, as was granulocyte colony stimulating factor for all high risk patients.
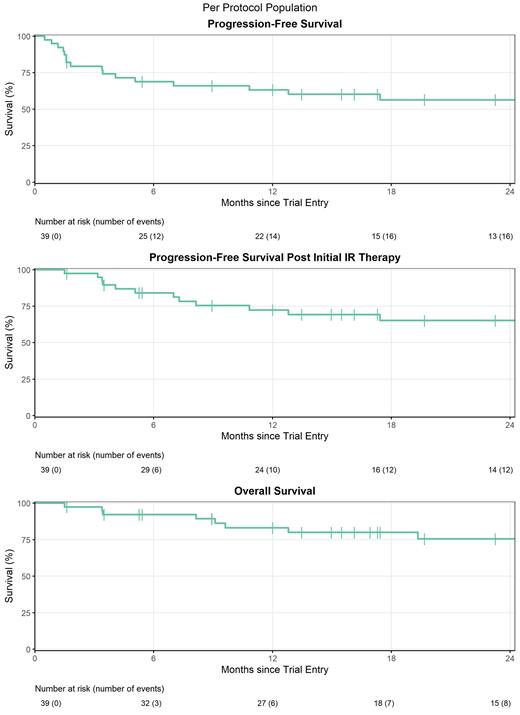
The primary outcome was CR assessed by interim CT. A CR rate of ≥40% was considered to be of interest, with an unacceptable CR rate set at ≤25%. Following a Simon's 2 stage design, 38 evaluable patients would need to be recruited, with at least 12 achieving CR to indicate further investigation is warranted. Secondary outcomes included allocation to the low risk arm, progression free survival (PFS), post-IR PFS (excluding disease progression events during initial IR treatment), overall survival (OS), and treatment tolerability.
Results
Between January 2017 and March 2020, 39 patients were recruited from 17 sites; 22 male, median age 59 years (range 23 to 76), 28% with IPI 3-5. Transplanted organs were kidney (n=20), liver (n=13), heart (n=4) and lung (n=2).
Primary outcome analysis was based on the first 38 patients recruited. After initial IR treatment, 11 patients (29%, 95% confidence interval 15% to 46%) achieved a CR, not reaching the pre-specified threshold. All other analyses were based on the full set of 39 evaluable patients. An overall response (OR) by 7 weeks was seen in 25 patients , comprising 12 CR and 13 PR. Sixteen patients (41%) were subsequently allocated to the low risk arm, 23 (59%) to high risk. , 26 patients (67%, 50% to 81%) achieved an OR (22 CR, 4 PR).
After a median follow up of 24 months, 1 year and 2 year outcomes are: PFS 63% (50% to 81%) and 56% (42% to 75%); post-IR PFS 72% (59% to 89%) and 65% (51% to 84%); OS 83% (72% to 96%) and 75% (62% to 92%).
Serious adverse events were most commonly infective (38%), and during IR-CHOP therapy (56%). Other common SAEs were gastrointestinal (31%) and haematological (9%). Two patient deaths due to sepsis and Pneumocystis pneumonia were related to treatment, 6 of the 7 further deaths were due to lymphoma.
Conclusions
Adding ibrutinib to rituximab for the initial treatment of PTLD did not result in a sufficiently high CR rate to warrant further investigation. Survival outcomes (PFS and OS) in our study are similar to those reported previously for rituximab with or without CHOP chemotherapy using a sequential risk stratified treatment approach. Of interest, in comparison to previous reports, a greater proportion of patients (41%) were allocated to low risk and continued IR therapy, thus avoiding cytotoxic chemotherapy. Therefore, the incorporation of novel agents in PTLD management merits further investigation to reduce treatment-related toxicity and improve survival.
Chaganti: Novartis: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Celgene-BMS: Consultancy, Honoraria, Other: Travel support; Atara Bio: Consultancy, Honoraria, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Janssen: Research Funding; Incyte: Honoraria, Speakers Bureau. Cwynarski: Gilead: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Atara: Consultancy; Celgene: Consultancy; Takeda: Consultancy, Other: travel to scientific conferences, Speakers Bureau; Kite, a Gilead Company: Consultancy, Other: travel to scientific conferences, Speakers Bureau; Janssen: Consultancy, Other: travel to scientific conferences; Roche: Consultancy, Other: travel to scientific conferences, Speakers Bureau; BMS/Celgene: Other: travel to scientific conferences. Fox: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Other: speaker fees. McKay: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Other: Travel to scientific conferences; KITE: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences; Janssen: Honoraria, Other: Travel to scientific conferences; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Paneesha: Janssen: Honoraria; Gilead: Honoraria; Bristol Myers Squibb: Honoraria; AbbVie: Honoraria; Roche: Honoraria; Celgene: Honoraria. Collins: Beigene: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Pfizer: Honoraria; Celgene: Research Funding; Amgen: Research Funding; AstraZeneca: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celleron: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck Sharp & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Speakers Bureau. Davies: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel to scientific conferences, Research Funding; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma/AstraZeneca: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; BioInvent: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel to scientific conferences, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees. Menne: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite/Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grant, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Astra Zeneca: Research Funding; Jazz: Other: Travel grants; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Bayer: Other: Travel grants; Kyowa Kirin: Other: Travel grants; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Atara: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Honoraria for Lectures; Roche: Other: Honoraria for Lectures.
Ibrutinib for PTLD.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal